 |
 |
 |
| Home | More Articles | Join as a Member! | Post Your Job - Free! | All Translation Agencies |
|
|||||||
|
|
An Update on the EU’s In-Vitro Diagnostic (IVD) Directive
New European Union legislation completing the Medical Device Directive is due to come into force at year’s end, which is bringing added challenges to product compliance in this field. This article looks at the implications of this complex Directive for the multilingual information management of product labeling in different countries and recommends relevant strategies.  New European Union legislation completing the Medical
Device Directive is due to come into force at year’s
end, which is bringing added challenges to product compliance
in this field. This article looks at the implications
of this complex Directive for the multilingual information
management of product labeling in different countries
and recommends relevant strategies.
New European Union legislation completing the Medical
Device Directive is due to come into force at year’s
end, which is bringing added challenges to product compliance
in this field. This article looks at the implications
of this complex Directive for the multilingual information
management of product labeling in different countries
and recommends relevant strategies. Localizing products for international markets is a challenging process, and it can be even more challenging for Life Sciences companies given the rules and regulations governing medical products. The primary set of regulations for Life Sciences companies to consider is the Medical Device Directive (MDD). The first two stages of the MDD were the Active Implantable Medical Device Directive, which had an implementation deadline of January 1, 1995 and the Medical Device Directive, which had an implementation deadline of June 13, 1998. The final stage of the MDD is the In-Vitro Diagnostics Directive (IVDD). One of the requirements that the IVDD specifies is that manufacturers’ comply with set language standards regarding instructions-for-use documents and labeling. The IVD Directive, at its core, is purposed to ensure the safety, quality, and performance of In Vitro Diagnostic (IVD) products. It is extremely important for IVD product manufacturers to understand that after December 7, 2003, all IVD products must be CE (Conformity European) marked or they will be prohibited from being sold in the European Union (EU). Any product lacking the CE Mark after the deadline will be blocked from entry into the EU. The IVD Directive applies to reagents and reagent products, calibrator materials or instruments including specimen receptacles intended by the manufacturer for the in-vitro examination of human tissue, blood or fluid samples for the purpose of providing information about a patient’s state of health. As stated earlier, compliance to the directive includes language translation of labeling, user instructions, or other key information into local languages as dictated by each EU Member State. Today, the European Union includes 15 Member States: Austria, Belgium, Denmark, Finland, France, Germany, Greece, Great Britain, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, and Sweden. It is also worth noting that in 2004, an additional ten countries will likely join the EU—with more to be added in 2007! Companies are now faced with the dilemma of providing the appropriate and required information in a multilingual format while making the information readable on a variety of label sizes. The use of internationally recognized symbols as a substitute for text has been actively promoted to provide users with a uniform method of obtaining product information. Yet many diagnostics companies are struggling with the issues that surround multilingual label design and international symbols. For example, the size of many labels will need to increase in order to support text expansion in the target language. “In some cases the English text may need to be rewritten with an eye towards brevity in order to support reduced space available for foreign language translation,” said Welocalize Project Manager, Deniz Gungen. Some of the standards referenced by the Directive include EN 980 (medical device symbols), EN 375 (professional use labeling), EN 376 (self-testing labeling), EN 1658 (marking of IVD instruments) and ISO 15223 (medical device symbols). Exceptions among the EU Member States further complicate matters, since there are differences in how each Member State adopts the regulation. Some countries provide exemptions to local language requirements, while others call for strict adherence to the Directive and may actually require that products be in several languages. Being informed about the national differences enables companies to determine if participation in a particular market is justified. (Please refer to the table below for language requirements for a particular country.) 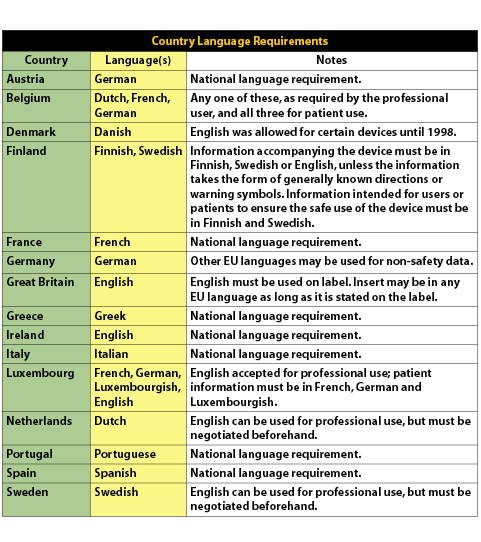
Welocalize also recommends the use of specialized consultants for the more complex regulatory, clinical and compliance issues. “As only one example, ALQUEST Inc. is a full-service Contract Research Organization offering regulatory, clinical and compliance services for medical devices, biologics, and combination products,” said Welocalize Business Development Manager, Mitch Bossart. CE marking IVD products for Europe is
a complex task. And there is confusion about how translation
requirements are related to the CE Mark. Simply stated,
the CE Mark is a European regulatory community sign
and symbolizes the compliance of the product with
the regulations relating to safety, public health,
consumer protection and the environment. Some of the
basic steps to achieve CE Marking are listed here.
For more detailed regulatory information on these
steps, please go to:
The IVDD was created to establish unified compliance standards throughout the EU to ensure the safety, quality and performance of in vitro diagnostic medical devices. Ultimately, this will make compliance easier and more efficient, since compliance will be harmonized across all EU Member States. However, it will take time and qualified resources to achieve successful compliance. is the President & CEO of Welocalize and leads the company's Life Sciences Localization/Translation practice. He can be reached at 800-370-9515 or smith.yewell@welocalize.com.
Reprinted
by permission from the Globalization Insider,
29 July 2003, Volume XII, Issue 3.3. Copyright the Localization Industry Standards Association (Globalization Insider: www.localization.org, LISA: www.lisa.org) and S.M.P. Marketing Sarl (SMP) 2004
E-mail this article to your colleague! Need more translation jobs? Click here! Translation agencies are welcome to register here - Free! Freelance translators are welcome to register here - Free! |
|
|
Legal Disclaimer Site Map |